Life Science &
Clinical Trials
Healthcare
Logistics for Clinical and Precinical Shipments
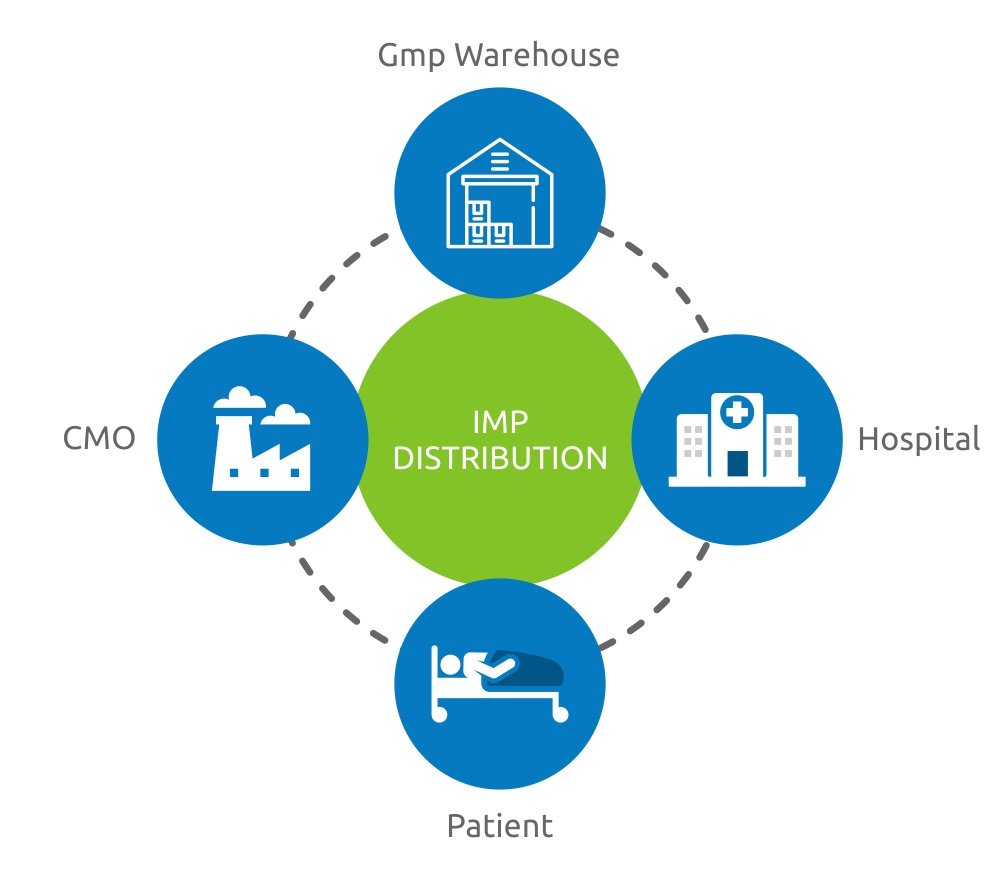
Since the beginning, PHSE has always dealt with biological and IMP transports (investigational medical products), that is biopharmaceutical products still not authorized by the related authority or clinical trials for laboratory use.
We provide logistics solutions from the “early research & development” and safeguard your biological samples and reagents. Api/bulk and experimental pharmaceuticals are our daily mission.
The strict temperature conditions and complex customs /normative procedures require our consultancy and assistance for:
- Legal and customs matters
- Cool chain knowledge
- The best packaging solution
- Temperature loggers management
- Quality control & Gdp protocols
- Global reach and local knowledge
Moreover, PHSE has a long-term experience cryogenic transportations with its dry shippers, very often used by the cellular therapy, regenerated medicine and assisted reproduction.
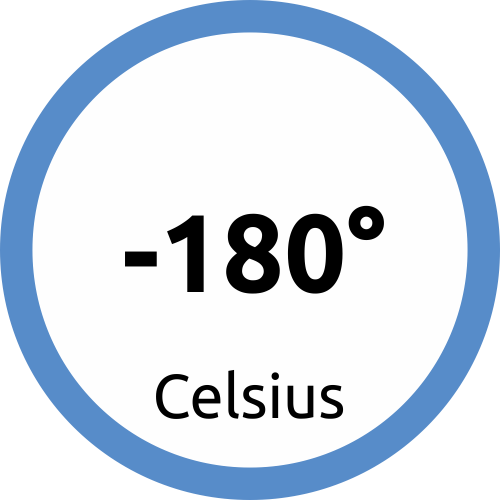
Cryogenic

Frozen

Refrigerated

Ambient

Controlled Ambient

uncontrolled
CLINICAL STUDIES
CLINICAL SHIMPENTS (biologicals/IMP)
AVERAGE OF SHIPMENTS WITH DATALOGGER
TRANSIT TIME 80% REQUIRES
Clinical Trials Logistics
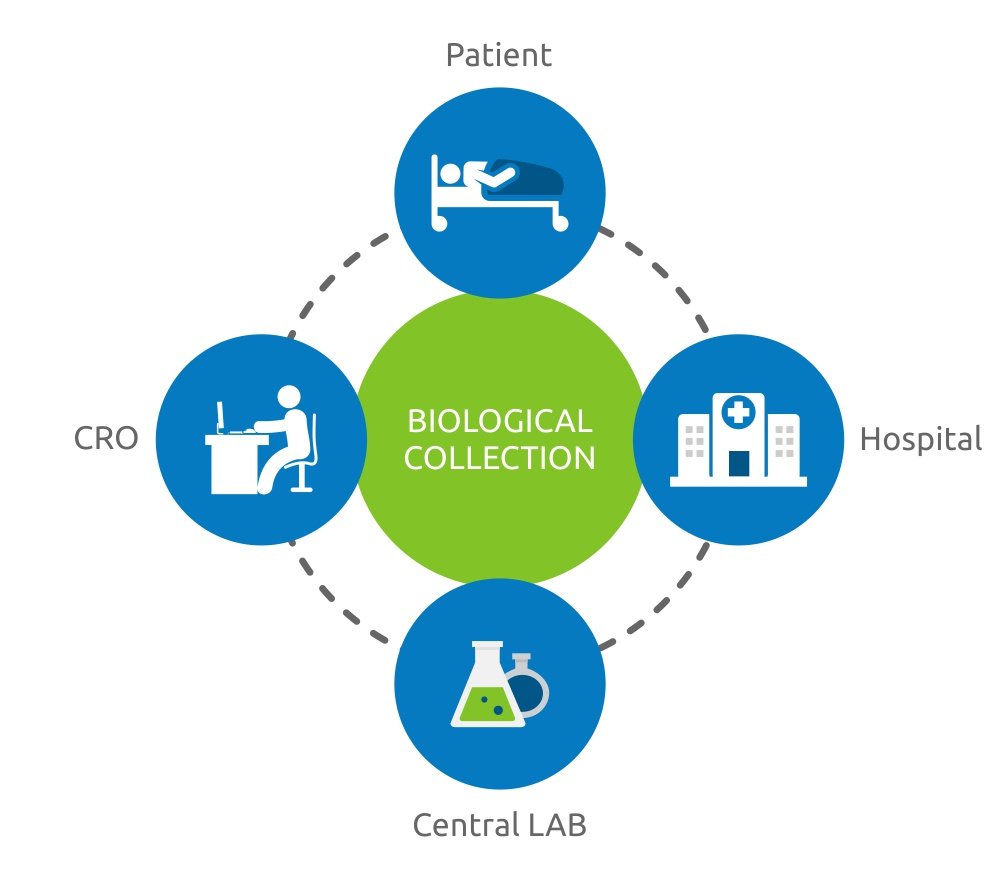
A clinical trial is more than a simple shipment. Handling with different shipments in different countries is a very complex activity. In Phse, just a person handles with different critical processes, such as:
- Planning and drafting the clinical protocol with all the logistics information of the shipment
- Monitoring the stock of the packaging/datalogger and their expiration date
- Handling the clinical site and the coordination between CRO, shipper and LAB
- Taking over the orders (by emails/phone/web)
- Handling the possible returns (expired product)
- In case of GMP handling the batch (label design, label printing and barcode)

In all these cases, transport must follow the clinical protocol requests (i.e. temperature, transit-time, and packaging) in order to assure integrity of the products (safety and stability) and safety of the patients.
PHSE has a deep knowledge of:
- Normative and customs procedures for each Country involved;
- Logistics processes based on proactivity and predictability of possible delays;
- Cool chain management
Clinical trials logistics has become decisive for clinical studies. Different climate conditions, different rules must not involve the cool chain. That is why the temperature monitoring is an outstanding aspect.
Tra gli studi clinici gestiti:
- 50% circa in ghiaccio secco
- 40% circa 2/8C e 10% ambient.
Tra le home patient deliveries:
- 80% circa 2/8C
AGRI-SCIENCE SERVICES
We are specialized also in the agricultural biotechnology logistics.
Due to the expected growing of world population up to 9, 6 milliards within 2050, researchers will have to find solutions to increase food productions, trying not to affect the environment.
PHSE helps their job furnishing complete and fast assistance, having the possibility to transport pallets at +2°/+8°C, in dry ice or either at ambient temperature.
We can provide also packaging, normative and customs assistance.
SHIPMENTS WE DEAL WITH
- Seeds
- plant extracts
- enzymes
- dry leaves/tissue samples
- bacteria samples
- pollen
- residuals samples
- fruits and vegetables as a whole or mashed
- herbicides and pesticides
FIELD SITES SERVICES
- Global site assistance
- Normative guide
- Furnishing of packaging and refrigerants
- Updates step-by step
- Customs clearance